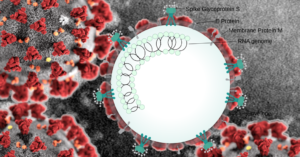
With the U.S. Food and Drug Administration’s approval for phase two trials, researchers are hoping that the Israeli drug Aviptadil, will be able to eliminate the so-called Acute Respiratory Distress Syndrome (ARDS), the condition responsible for the deaths of about 50% of all COVID-19 patients.
By i24NEWS
The U.S. Food and Drug Administration on Sunday has cleared NeuroRx, a US-Israeli pharmaceutical company, and Relief Therapeutics, a Swiss drug development company, for phase two trials for a drug that could take on a deadly condition associated with COVID-19.
The two companies announced that the FDA issued a “study may proceed” letter — which does not amount to a full-fledged drug approval — for the substance to be tried on COVID-19 patients.

An ECMO (extracorporeal membrane oxygenation) unit at Sheba Medical Center’s new Corona Critical Care Unit (CCCU). An ECMO machine pumps and oxygenates a patient’s blood outside the body, allowing the heart and lungs to rest. – Photo: Courtesy, Sheba Medical Center (Israel)
The drug in question, Aviptadil, is a synthetic form of a neuropeptide hormone that works to enable communications between neurons in the human nervous system.
The researchers are hoping that Aviptadil will be able to take on the so-called Acute Respiratory Distress Syndrome (ARDS) — a condition responsible for about 50% of COVID-19 fatalities.
RELATED:
- Palestinians forced to stop producing fake coronavirus masks, hand sanitizer
- Israel Ranks 1st Worldwide in Safety Amid COVID-19 Pandemic
- Israeli division CEO gives away patents to manufacture ventilators
ARDS is a respiratory system failure induced by a rapid and severe lung inflammation, with breath shortness — a symptom COVID-19 is widely associated with — among its key signs.
It effectively brings to a halt the oxygen and carbon dioxide exchange in the patient’s lungs, necessitating the use of an artificial lung ventilation machine.
View Ynet publication at:
https://www.ynetnews.com/health_science/article/B1SdAHxw8
ISRAEL
‘as a light unto the nations’





 Israeli New Shekel Exchange Rate
Israeli New Shekel Exchange Rate